Full link here:
https://www.creative-diagnostics.com/sars-cov-2-coronavirus-multiplex-rt-qpcr-kit-277854-457.htm
SARS-CoV-2 Coronavirus Multiplex RT-qPCR Kit (CD019RT)
Regulatory status: For research use only, not for use in diagnostic procedures.
COA
SDS Online Inquiry Add to Basket
Specificity
non-specific interference of Influenza A Virus (H1N1), Influenza B Virus (Yamagata), Respiratory Syncytial Virus (type B),
Respiratory Adenovirus (type 3, type 7), Parainfluenza Virus (type 2), Mycoplasma Pneumoniae, Chlamydia Pneumoniae, etc.
Species Reactivity
Human
Application
Qualitative
Size
100T
StorageAll reagents should be stored at -30°C~-15°C with protection from light.
The reagents are stable for 12 months when stored at the recommended condition.
The expiration date will not change if the kit is opened and stored at the recommended condition.
The expiration date will not change if the kit is transported with ice-packs for 4 days and/or treated with 10 freeze-thaw cycles.
Intended Use
This product is intended for the detection of 2019-Novel Coronavirus (2019-nCoV). The detection result of this product is only for clinical reference, and it should not be used as the only evidence for clinical diagnosis and treatment.
Principles of Testing
This product is a dual-color multiplex fluorescent probe-based Taqman® RT-qPCR assay system. The Taqman fluorescent probe is a specific oligonucleotide based on a reporter-quencher mechanism. For each probe, the 5′-end is labeled with a fluorophore, while the 3′-end was labeled with a quencher. When the probe is intact, the fluorescence emitted by the fluorophore is absorbed by the quencher, and no fluorescent signal is detected. However, during amplification of the template, the probe will be degraded due to the 5′-3′ exonuclease activity of Taq DNA polymerase, and the fluorescent reporter and the quencher are cleaved and separated, then a fluorescent signal can be detected. The generation of each molecular amplicon is accompanied by the generation of a fluorescent signal. Real-time monitoring of the entire PCR process can be assessed by monitoring the accumulation of fluorescent signals.
This product provides dual-detections of two independent genes of 2019-nCoV in a single tube. Specific primers and probes were designed for the detection of conserved region of 2019-nCoV’s ORF1ab gene and N gene, respectively, avoiding non-specific interference of SARS2003 and BatSARS-like virus strains.
Detection Limit
500 copies /mL.
Reagents And Materials Provided
1. Detection Buffer (900 μL × 2 tubes), including Buffer, dNTPs, Primers, Probes.
2. Enzyme Mix (200 μL × 1 tube), including RNase Inhibitor, UDG, Reverse Transcriptase, Taq DNA polymerase.
3. Positive Control (200 μL × 1 tube), plasmid containing target fragment.
4. Negative Control (500 μL × 1 tube), DEPC-Treated Water.
Note: Do not mix the components from different batches for detection.
Materials Required But Not Supplied
Real-time PCR instrument with both FAM and TEXAS RED channels,
such as ABI7500, ABI Q3, ABI Q6, Roche LightCycler480, Bio-Rad CFX96.
Specimen Collection And Preparation
1. Suitable specimen type: upper respiratory specimen (including nasal swabs, nasopharyngeal swabs / aspirates / washes, and sputum) and lower respiratory specimen (including respiratory aspirates, bronchial washes, bronchoalveolar lavage fluids, and lung biopsy specimens).
2. For detailed methods of specimen collection, please refer to the protocol in the “Microbiology Specimen Collection Manual”.
3. The collected specimen should be used for detection within the same day. Otherwise, please store the specimen as follows:
Store at 2°C – 8°C for no more than 24 hours;
Store at < -20°C for no more than 10 days;
Store at < -70°C for long-term, avoiding repeated freeze-thaw cycles.
4. The specimen should be transported using sealed foam box with dry ice.
Specimen Preparation
The samples should be extracted according to the corresponding requirements and procedures of viral RNA extraction kits. The extracted RNA can be directly used for detection. If the extracted RNA is not used for detection immediately, please store the RNA at below -70°C, avoiding repeated freeze-thaw.
Reagent Preparation
Thaw the required reagents, mix by shaking, and centrifuge briefly before use. Prepare the mixture in a RNase-free centrifuge tube as follows:

Note: It is recommended to set both negative and positive controls for each test.
Mix the above mixture thoroughly, and make aliquots of 20 μL into different PCR reaction tubes. Then, move to the Specimen Preparation Area.
Assay Procedure
1. Template Addition (Specimen Preparation Area)
Add 5 μL of Negative Control (no extraction required), 5 μL of Positive Control (no extraction required), and 5 μL of extracted RNA from specimen to different PCR reaction tubes which contained 20 μL of PCR mix.
2. RT-PCR Amplification (Detection Area)
Put the reaction tubes on a PCR instrument, setup and run the following cycling protocol:
Settings of detection fluorescence: ORF1ab gene (FAM), N gene (TEXAS RED / ROX). Please set the internal reference parameter of fluorescence of the instrument to “None”. For example: for ABI series instruments, please set “Passive Reference” to “None”.
3. Data Analysis (refer to Instrument User Manual)
Take ABI7500 as an example: after the qPCR reaction, the results were saved automatically. According to the analyzed image, please
adjust the Start value, End value, and Threshold value of the Baseline (Start value: 3 ~ 15; End value: 5 ~ 20; Threshold value could be set in the Log window, and the threshold line should be in the exponential phase of the amplification curve; the amplification curve of the negative control should be straight or below the threshold line). Click “Analysis” to obtain the analysis result automatically, and read the detection result in the “Report” window.
Quality Control
The result is valid if ALL the above criteria is met. Otherwise, the result is invalid.
Interpretation Of Results
If the criteria of QUALITY CONTROL is met, analysis the data of sample as follows:
Precision
Using two cases of high and low positive quality products to test for 10 consecutive times, the CV of their Ct values is ≦5%.
Precautions1. Please read this manual carefully before beginning the experiment, and strictly follow the instructions.
2. This product should be only used by trained labor personnel in safety protected laboratories and wear appropriate protective equipments.
3. This product should be protected from light. Please use sterile, DNasefree, and RNase-free tubes and tips during the detection.
4. The tested specimen of this product is regarded as infectious material. The operation and treatment should meet the requirements of the local regulations and laws.
Limitations1. The detection result of this product is only for clinical reference, and it should not be used as the only evidence for clinical diagnosis and treatment. The clinical management of patients should be considered in combination with their symptoms/signs, history, other laboratory tests and treatment responses. The detection results should not be directly used as the evidence for clinical diagnosis, and are only for the reference of clinicians.
2. The detection result can be affected by operations, including specimen collection, storage and transportation. False negative result may occur if there is any mistakes in the operation. Cross contamination during specimen treatment may lead to false positive result.
3. The detected target sequences of this products are the conservative region of 2019-nCoV’s ORF1ab gene and N gene. However, target sequence variations may lead to false negative result.
Have you cited CD019RT in a publication? Let us know and earn a reward for your research.
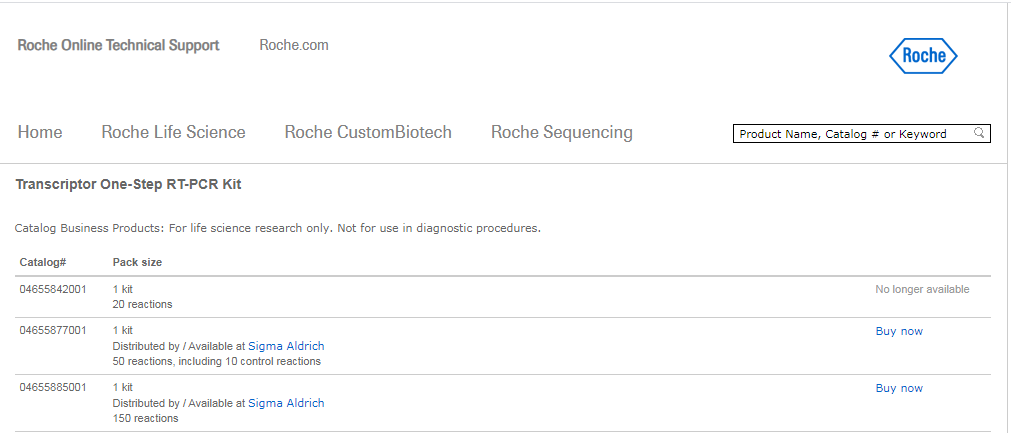
http://technical-support-test.roche.com/product.aspx?productId=3.6.17.2.7.2

https://altona-diagnostics.com/en/products/reagents-140/reagents/realstar-real-time-pcr-reagents/realstar-sars-cov-2-rt-pcr-kit-ruo.html